nitro-Grela

Nazwa chemiczna:
(1,3-dimesitylimidazolidin-2-ylidene)dichloro(2-isopropoxy-5-nitrobenzylidene)ruthenium(II)
Numer produktu: AS2032
Numer CAS: 502964-52-5
Numer patentu: PCT/EP2003/011222
Zapytaj o dostępnośćOpis
Uniwersalny katalizator zapewniający dobre wyniki w szerokim spektrum reakcji. Zapewnia wysoką selektywność w reakcjach metatezy krzyżowej (CM) pomiędzy olefinami typu 1 (szybka homodimeryzacja) i partnerami z niedoborem elektronów. Znajduje zastosowanie w reakcjach metatezy z substratami posiadającymi wiązanie podwójne zatłoczone sterycznie.
Porównanie z konkurencyjnymi produktami:
- RCM: Możliwe jest przeprowadzenie reakcji w niższej temperaturze (0 °C). Hoveyda-Grubbs II wymaga wyższej temperatury.
- CM: nitro-Grela pozwala na przeprowadzenie reakcji w niższej temperaturze, przy niższym zużyciu katalizatora niż Grubbs-II i Hoveyda-Grubbs II. Pożądane produkty otrzymuje się z wyższą selektywnością.
- En-yn: Użycie nitro-Grela daje wyższą konwersję przy 2,5 razy niższym zużyciu katalizatora niż w przypadku Hoveyda-Grubbs.
Właściwości chemiczne
Wzór chemiczny:
C31H37CI2N3O3Ru
Masa cząsteczkowa:
671.62 g/mol
Stan fizyczny:
proszek
Barwa:
zielony
Informacje o użytkowaniu
Wysoka stabilność pozwala na używanie katalizatora na powietrzu.
Informacje o przechowywaniu
Przechowywanie długoterminowe (>1 tydzień):
Przechowuj w atmosferze gazu obojętnego w chłodnym miejscu (zalecana temp. 2 - 8 °C).
Przechowywanie krótkoterminowe (<1 tydzień):
Przechowuj w atmosferze gazu obojętnego w temperaturze otoczenia.
Zastosowania
Badania własne Apeiron Synthesis:
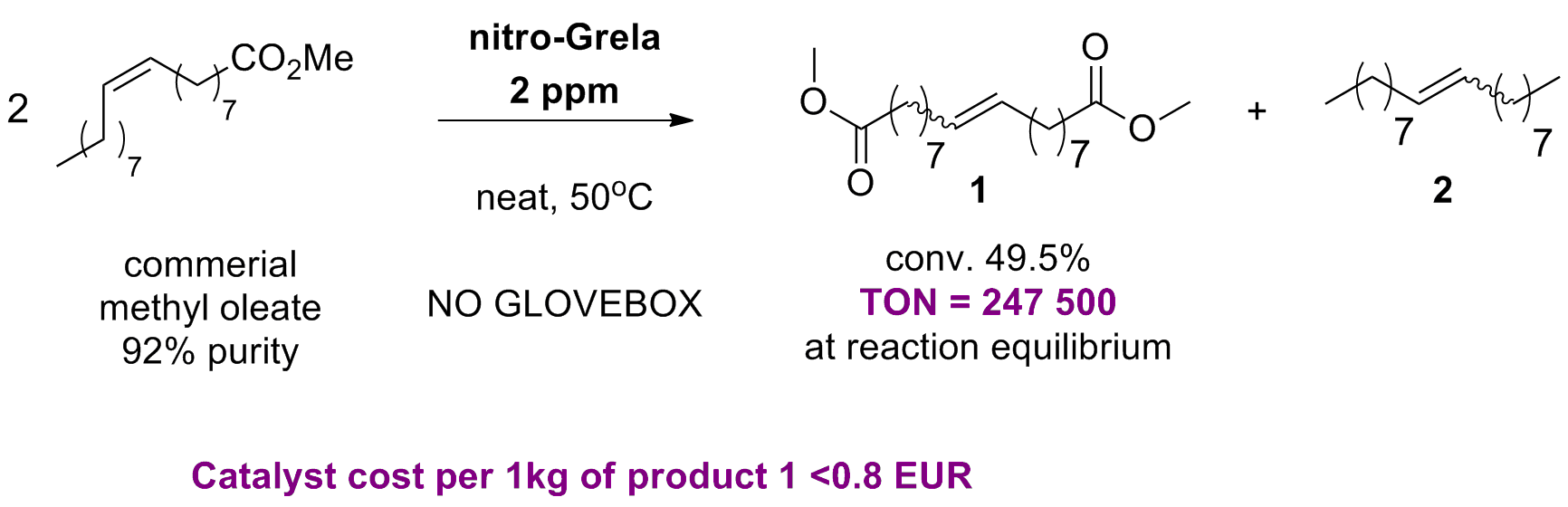
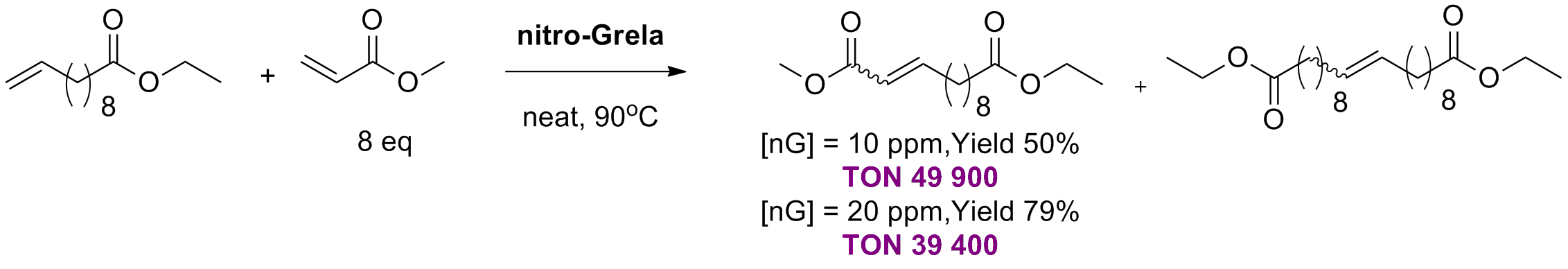
K. Kaczanowska, B. Trzaskowski, A. Peszczyńska, A. Tracz, R. Gawin, T. K. Olszewski, K. Skowerski “Cross metathesis with acrylates: N-heterocyclic carbene (NHC) versus cyclic alkyl amino carbene (CAAC)-based ruthenium catalysts, an unanticipated influence of the carbene type on efficiency and selectivity of the reaction” ChemCatChem, 12, 24, 6366-6374, (2020).
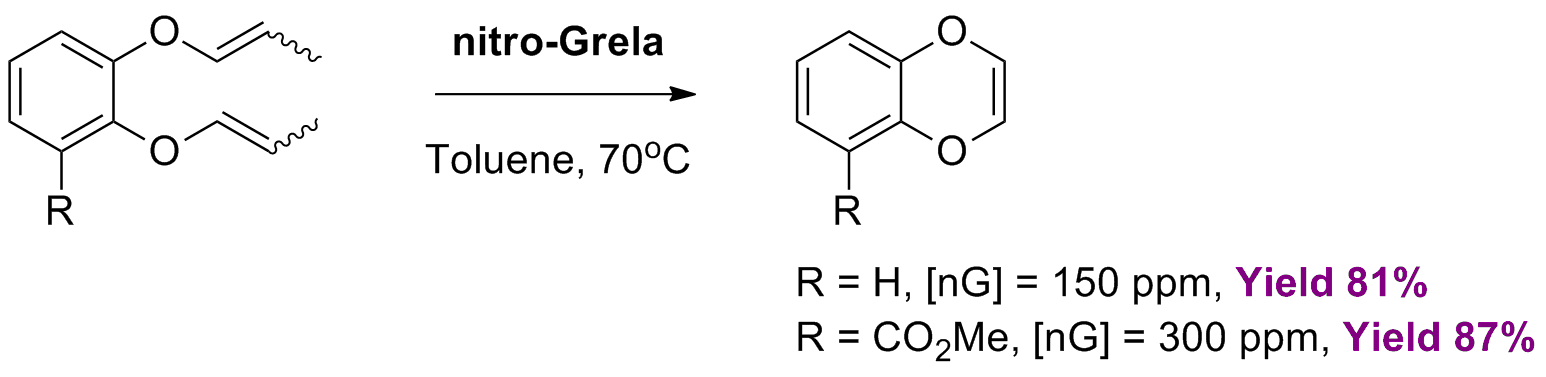
E. Chong, B. Qu, Y. Zhang, Z. P. Cannone, J. C. Leung, S. Tcyrulnikov, K. D. Nguyen, N. Haddad, S. Biswas, X. Hou, K. Kaczanowska, M. Chwalba, A. Tracz, S. Czarnocki, J. J. Song, M. C. Kozlowski, C. H. Senanayake “A Versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes” Chem. Sci., 10, 4339-4345, (2019).
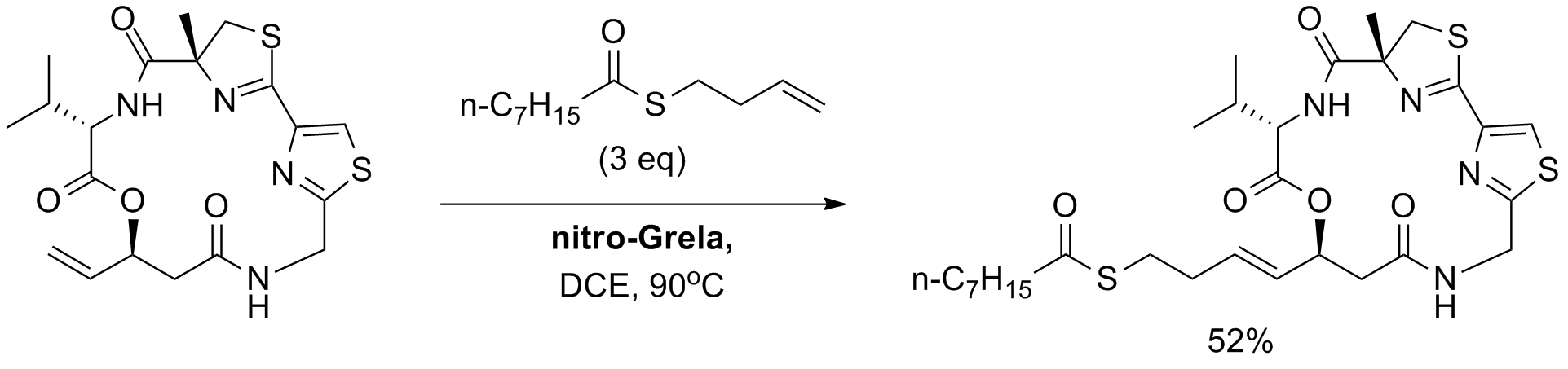
Q.-Y. Chen, P. R. Chaturvedi, H. Luesch “Process Development and Scale-up Total Synthesis of Largazole, a Potent Class I Histone Deacetylase Inhibitor” Org. Process Res. Dev., 22, 2, 190-199, (2018); B) T. Seiser, F. Kamena, N. Cramer “Synthesis and biological activity of largazole and derivatives” Angew. Chem. Int. Ed., 47, 34, 6483-6485, (2008).
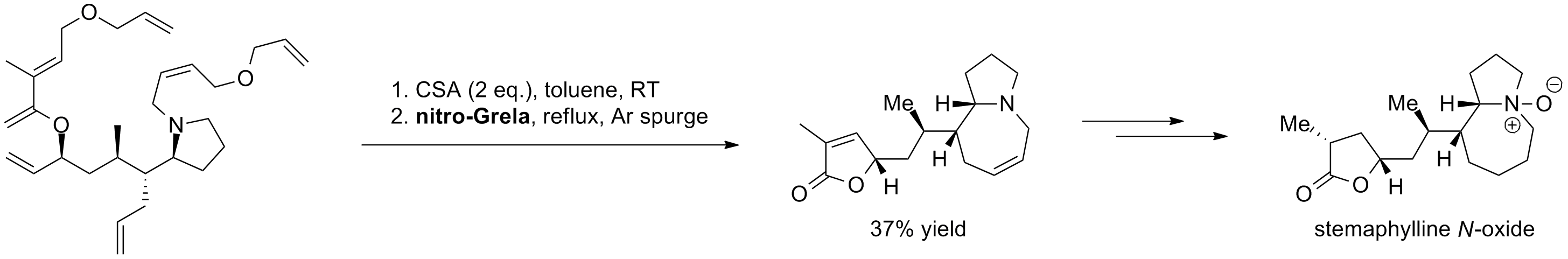
M. L. Schulte, M. L. Turlington, S. S. Phatak, J. M. Harp, S. R. Stauffer, C. W. Lindsley “Total Synthesis of Stemaphylline N-Oxide and Related C9a-Epimeric Analogues” Chem. Eur. J., 19, 36, 11847-11852, (2013).
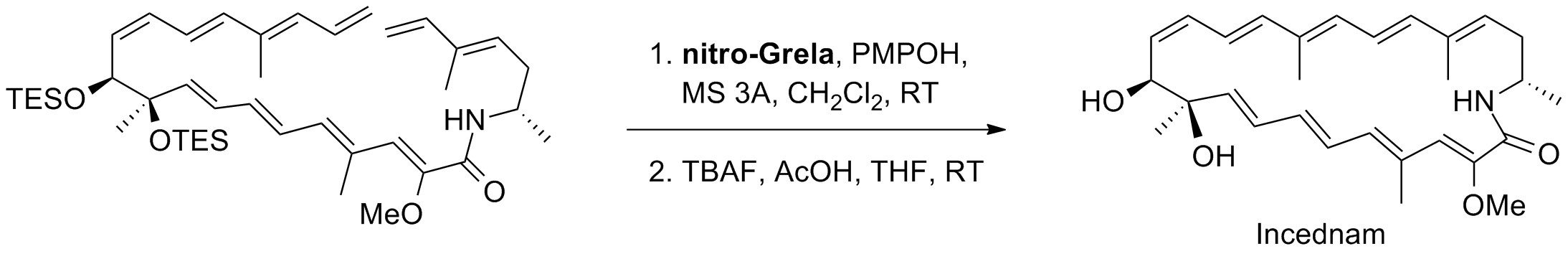
A. Takada, K. Uda, T. Ohtani, S. Tsukamoto, D. Takahashi, K. Toshima “Improved total synthesis of incednam” J. Antibiot., 66, 155-159, (2013).
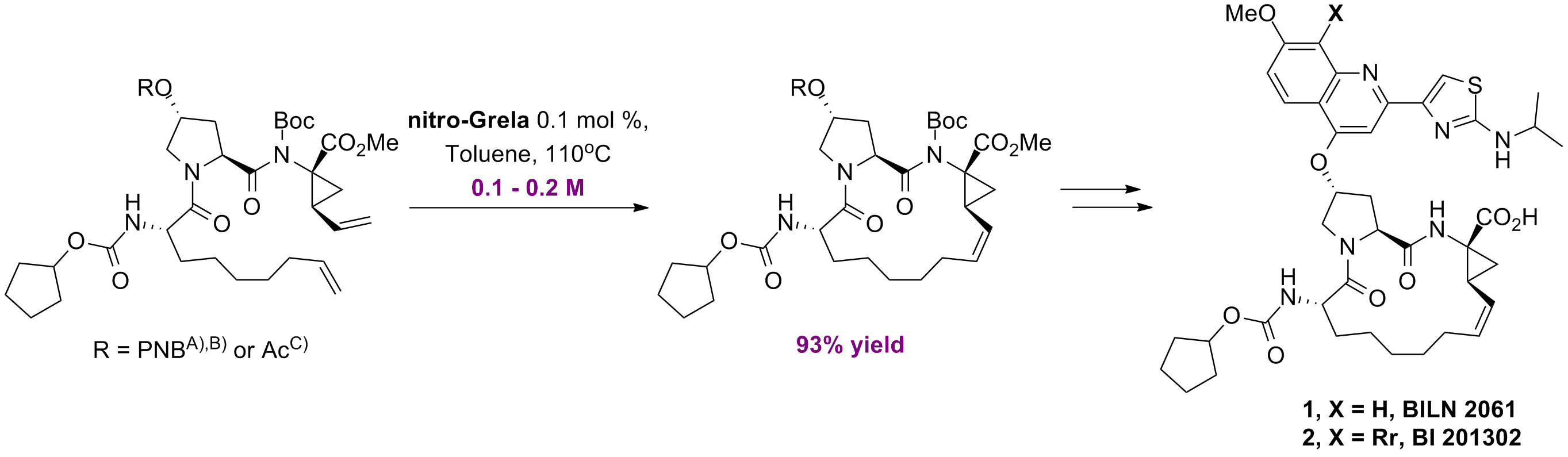
C. Shu, X. Zeng, M-H. Hao, X. Wei, N. K. Yee, C. A. Busacca, Z. Han, V. Farina, C. H. Senanayake “RCM Macrocyclization Made Practical: An Efficient Synthesis of HCV Protease Inhibitor BILN 2061” Org. Lett. 10, 6, 1303-1306, (2008); B) V. Farina, C. Shu, X. Zeng, X. Wei, Z. Han, N. K. Yee, C. H. Senanayake “Second-Generation Process for the HCV Protease Inhibitor BILN 2061: A Greener Approach to Tu-Catalyzed Ring-Closing Metathesis” Org. Process Res. Dev., 13, 2, 250-254, (2009); C) X. Wei, C. Shu, N. Haddad, X. Zeng, N. D. Patel, Z. Tan, J. Liu, H. Lee, S. Shen, S. Campbell, R. J. Varsolona, C. A. Busacca, A. Hossain, N. K. Yee, C. H. Senanayake “A Highly Convergent and Efficient Synthesis of a Macrocyclic Hepatitis C Virus Protease Inhibitor BI 201302” Org. Lett., 15, 5, 1016-1019, (2013).
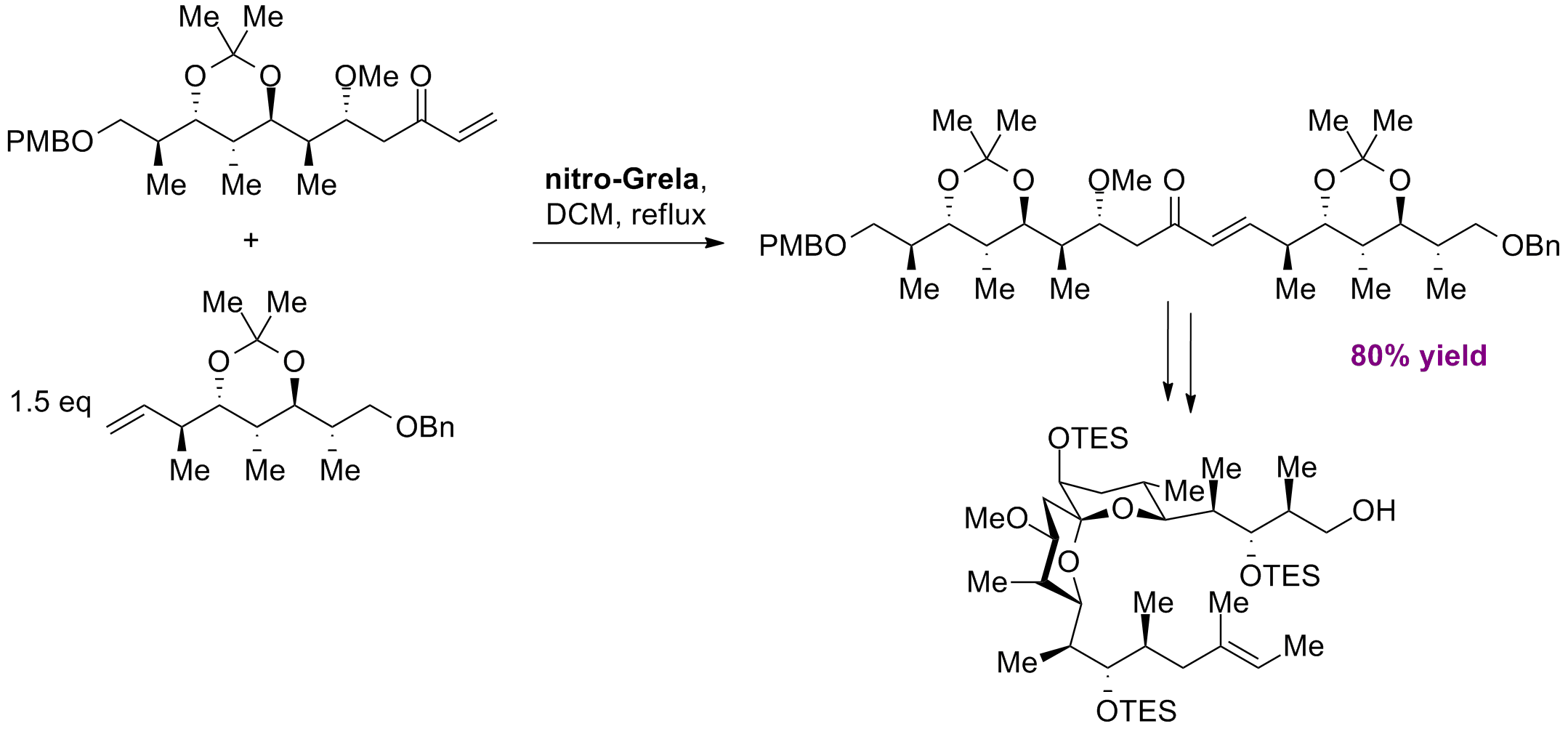
C. Gregg, C. Gunawan, A. Wai Yi Ng, S. Wimala, S. Wickremasinghe, M. A. Rizzacasa “Formal Total Synthesis of Spirangien A” Org. Lett., 15, 3, 516-519, (2013).
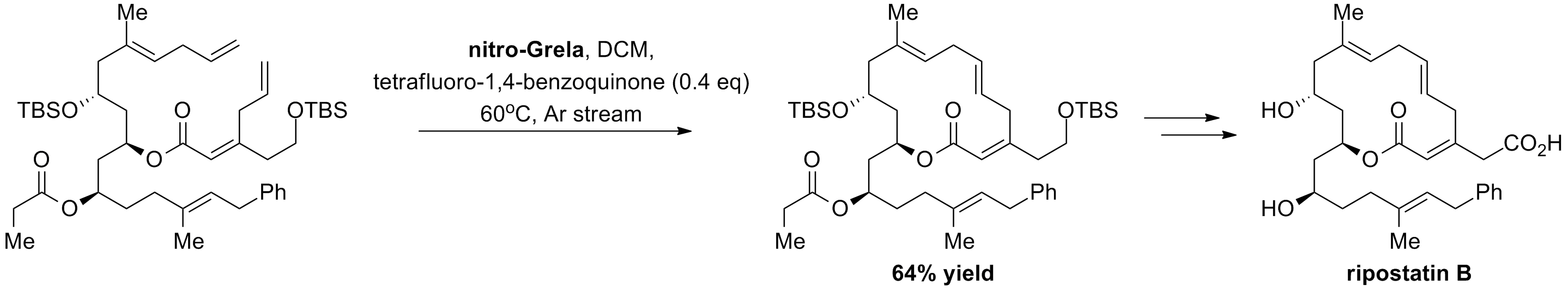
P. Winter, W. Hiller, M. Christmann “Access to Skipped Polyene Macrolides through Ring-Closing Metathesis: Total Synthesis of the RNA Polymerase Inhibitor Ripostatin B” Angew. Chen. Int. Ed., 51, 14, 3396-3400, (2012).
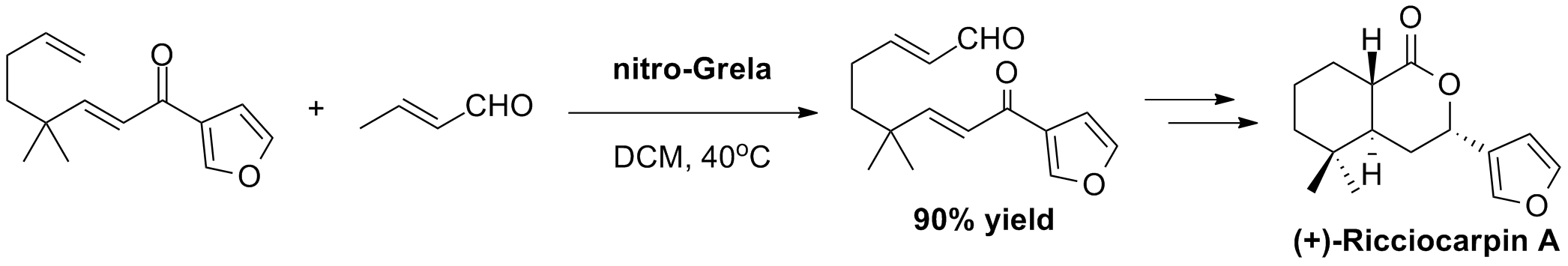
A. Michrowska, B. List “Concise synthesis of ricciocarpin A and discovery of a more potent analogue” Nat. Chem., 1, 225, (2009).
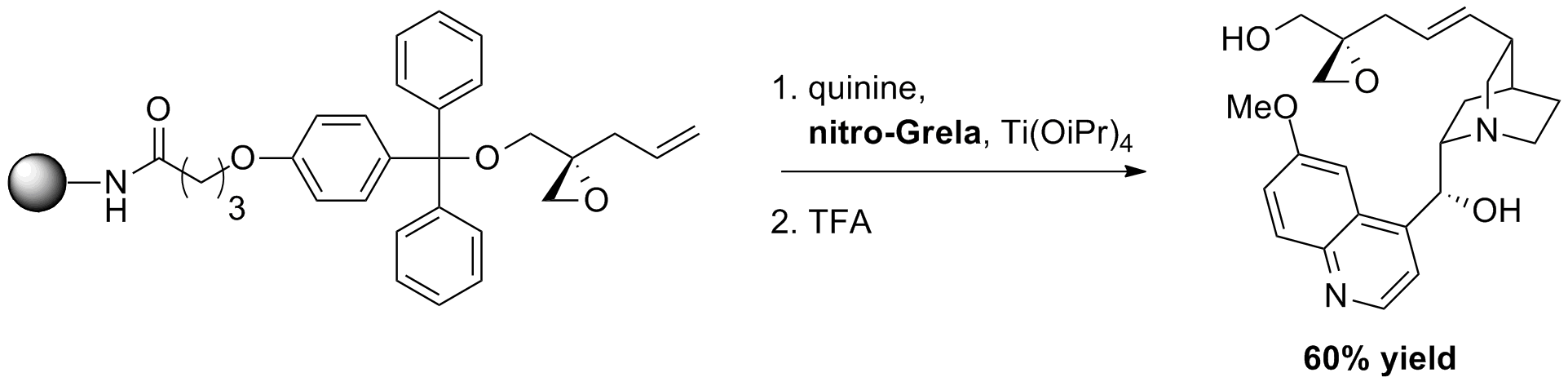
A. Garner, K. Koide “Solid-Phase Olefin Cross-Metathesis Promoted by a Linker” Org. Lett., 9, 25, 5235-5238, (2007).
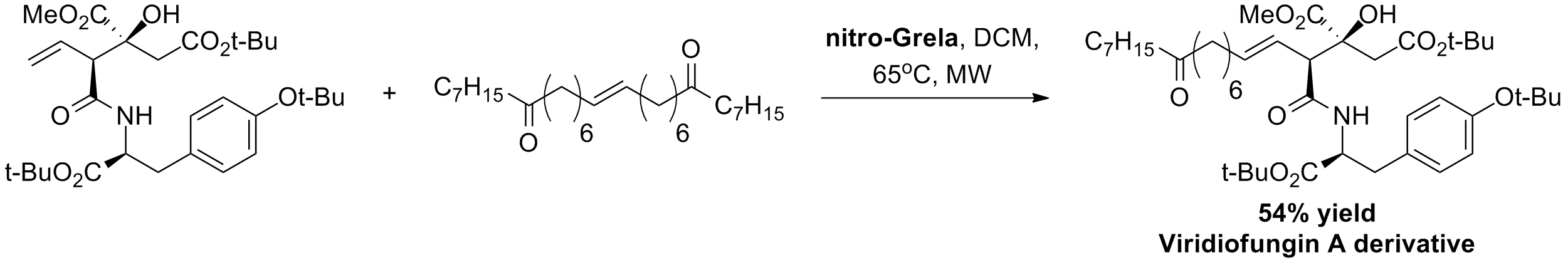
S. M. Goldup, C. J. Pillkington, A. J. P. White, A. Burton, A. G. M. Barrett “A Simple, Short and Flexible Synthesis of Viridiofungin Derivatives” J. Org. Chem., 71, 16, 6185-6191, (2006).

B. J. Albert, A. Sivaramakrishnan, T. Naka, K. Koide “Total Synthesis of FR901464, an Antitumor Agent that Regulates the Transcription of Oncogenes and Tumor Suppressor Genes” J. Am. Chem. Soc., 128, 9, 2792-2793, (2006).
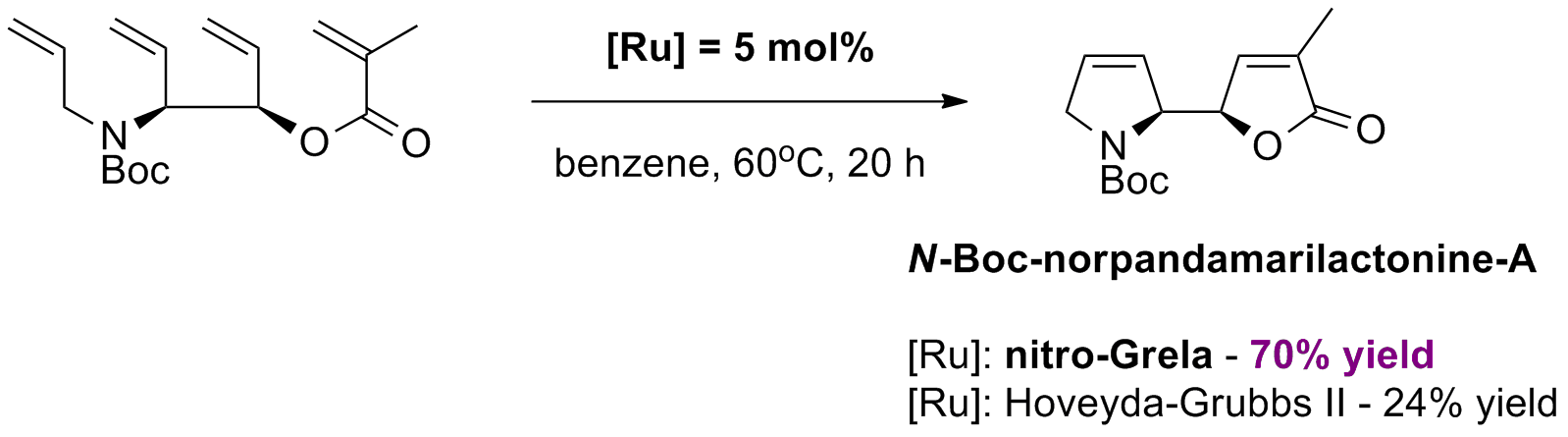
T. Honda, M. Ushiwata, H. Mizutani “Novel stereoselective synthesis of enantiopure (+)-N-Boc-norpandamarilactonine-A, the intermediate for pandamarilactonines” Tetrahedron Lett., 47, 35 6251-6254, (2006).
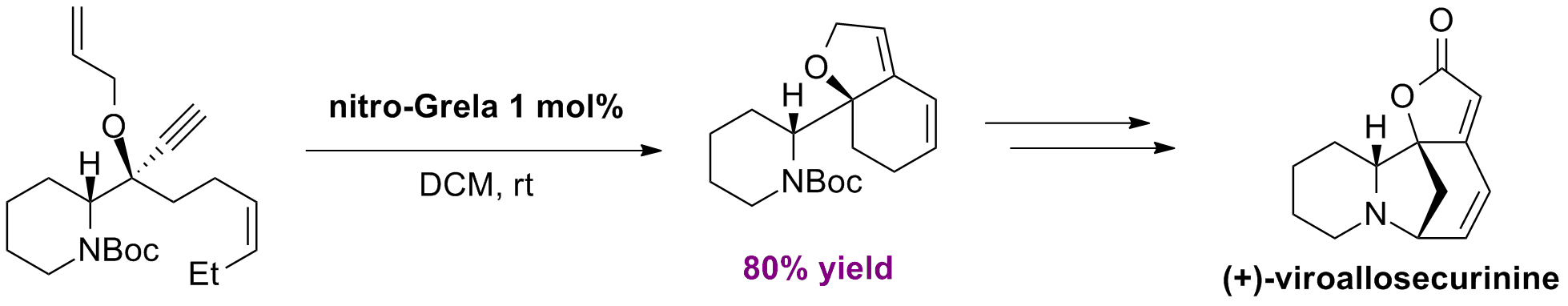
T. Honda, H. Namiki, M. Watanabe, H. Mizutani “First total synthesis of (+)-viroallosecurinine” Tetrahedron Lett., 45, 27, 5211-5213, (2004); B) T. Honda, H. Namiki, K. Kaneda, H. Mizutani “First Diastereoselective Chiral Synthesis of (-)-Securinine” Org. Lett., 6, 1, 87-89, (2004).

O. M. Demchuk, M. K. Pietrusiewicz, A. Michrowska, K. Grela “Synthesis of substituted P-stereogenic vinylphosphine oxides by olefin cross-metathesis” Org. Lett., 5, 18, 3217-3220, (2003).
Referencje
- K. Grela, S. Harutyunyan, A. Michrowska „A New Highly Efficient Ruthenium Catalyst for Metathesis Reaction” Angew. Chem. Int. Ed., 41, 4038 – 4040, (2002).
Pytania odnośnie produktu?
Europa
Agnieszka Roszyk
Sprzedaż i wsparcie techniczne
agnilwpk6eszka.roszyk@apeiron-synthesis.com
Tel.: (+48) 71 798 56 23
Ameryka Północna
dr Grażyna Szymańska
Global Business Development
grazyywna.szymanska@apeiron-synthesis.com
Tel.: (+1) 781 608 5859





