Sprawdź kolejne, interesujące zastosowanie naszego katalizatora AquaMet do pionierskiej syntezy niezabezpieczonych peptydów w wodzie!
Niedawno na łamach czasopisma Organic & Biomolecular Chemistry ukazał się artykuł naukowy S. Masuda i in., ukazujący wyniki badań autorów, dotyczących syntezy niezabezpieczonych peptydów w wodzie poprzez metatezę z zamknięciem pierścienia (RCM).
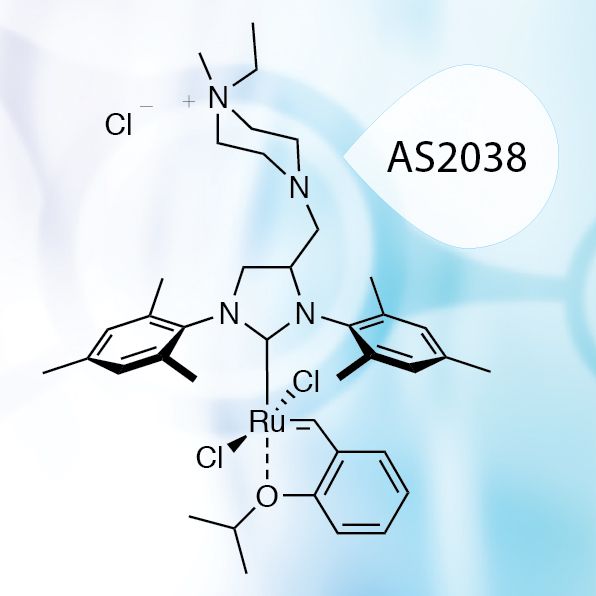
Choć RCM jest atrakcyjnym sposobem wytwarzania sztucznych, zaprojektowanych peptydów, to w artykule zatytułowanym „Ring-closing metathesis of unprotected peptides in water” po raz pierwszy doniesiono o możliwości przeprowadzenia syntezy niechronionych peptydów w wodzie w obecności jonów Mg(II), bez dodatku jakichkolwiek rozpuszczalników organicznych poprzez RCM, czego dokonano dzięki zastosowaniu rozpuszczalnego w wodzie katalizatora rutenowego – AquaMet, produkowanego przez Apeiron Synthesis.
Autorzy przeprowadzili optymalizację warunków reakcji i wykazali, że dodatek MgCl2 i/lub zastosowanie warunków kwasowych umożliwia wydajną reakcję RCM niezabezpieczonych peptydów modelowych i analogów oktreotydu w wodzie. Opisana metoda, z zastosowaniem naszego katalizatora może okazać się cennym narzędziem w syntezie biologicznie aktywnych, biomimetycznych peptydów.
Pełna wersja artykułu dostępna: tutaj